Catalog
Recently Viewed
CM13CTv2 - p3-Truncated Helper Phage
Technical Description
CM13CTv2 is a helper phage especially engineered for phage display. CM13CTv2 is a derivative of CM13 having a truncated version of the minor coat protein pIII bearing only the CT domain. PIII-CT is incorporated in virion capside, but cannot mediate bacterial transduction. Therefore, transduction will almost exclusively derive from fusion phage bearing a wild-type protein III, typically made by the phagemid (Fig. 1). The use of CM13CTv2 has been shown to result in more selective biopannings (1).
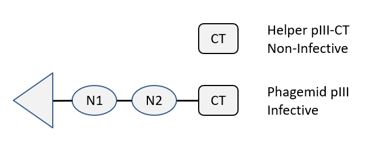
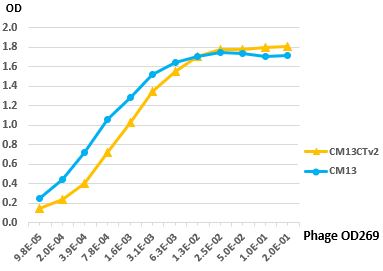
Figure 1. CM13CTv2 Display. CM13CTv2 helper phage contains a truncated copy of p3 expressing only the CT domain. The CT domain is not functional and cannot transduce bacteria. Therefore, only phage bearing a fusion protein with a full-length pIII are infective and are amplified after elution (Upper Panel). The variant v2 was selected for robust production, high infectivity and good display. A phage ELISA with virions displaying a Fab fragment produced with either CM13 or CM13CTv2 shows similar binding activities (Lower Panel).
Applications
-
Phage display.
For research use only; not intended for any animal or human therapeutic or diagnostic use.
Source
CM13CTv2 virions were isolated from the supernatant of infected E. coli providing the full-length p3 and purified by PEG precipitation.
Specifications
Composition: 50% glycerol TBS buffered.
Concentration: >2 x1012 cfu/ml; infectivity ~13%
Storage temperature: -20°C.
Product size: 0.5 ml.
Quality Control & Certification of Analysis
Virion concentration:
Phage DNA concentrations are determined by UV spectrophotometry and virion concentrations calculated based on the length of CM13CTv2 genome.
Phage titer:
CM13CTv2 preparations are serially diluted and mixed with TG1 cells aadna plaataes agar plates and incubated overnight at 37°C; the morning after plaques are counted. Controls include plates without virions and have no visible plaques.
Infectivity:
Infectivity is measured as cfu after transduction of TG1 cells. Pfu counts on pIII-supplemented bacteria are ~4-5 times higher.
Contamination check:
Phage preparations show no growth on 2xYT agar plates.
Certification:
Products meet all specifications.
Notes
Conditions for optimal transduction:
Always use bacteria freshly made from the night before; typically grow your phagemid-containing bacteria overnight in 2xYT medium from a single colony in the presence of ampicillin and glucose at 30°C or 37°C. Dilute the bacterial culture 1:20 v/v with fresh 2xYT medium and incubate for one hour at 37°C. Measure the absorbance at 600 nm, at best using a large 1 ml cuvette and a 1:5 or a 1:10 dilution; adjust to 0.5 OD. We recommend 1 µl of CM13CTv2 preparation per ml of culture as a good balance between not using too much helper and achieving a high level of superinfection; more helper may yield more phage but results are often inconsistent.
Immediately transfer the culture to a shaker and incubate for 30 min to 1 h at 37°C and 250 rpm; preincubation of helper and bacteria on the bench or at 37°C without shaking is unnecessary and often leads to a greater variability in phage yields; best superinfections are obtained at 37°C and 250 rpm. Then add kanamycin 50 µM, ampicillin 100 µM and IPTG 200 µM if you are using pADL-10b, pAK100 or pAK200 phagemid and lower the temperature to 30°C; harvest the phage after 8 h to o/n.
Influence of cell density:
An F+ bacterial strain containing the F plasmid is required for superinfection by CM13CTv2 virions. Removal of glucose, induction of the Lac promoter and expressing the fusion coat protein at possible toxic levels may cause the bacteria to stop growing; therefore, it is better to add the helper phage at the highest cell density possible. Inversely the presence of a large percentage of non-transduced bacteria can lead to low phage production; this problem is accentuated by a natural immunity to M13 superinfection caused by endogenous expression of full-length protein III, including expression by the phagemid. We have determined that the optimal cell density at which the helper phage is added to maximize phage production is at an optical density between 0.4 and 0.5 at 600 nm for TG1 and SS320 cells.
References
- Kramer RA, Cox F, van der Horst M, van der Oudenrijn S, Res PC, Bia J, Logtenberg T, de Kruif J. A novel helper phage that improves phage display selection efficiency by preventing the amplification of phages without recombinant protein. Nucleic Acids Res.. 2003;31(11):e59
Related Products



