Llama Single Domain Antibody Platform
Antibody Design Labs offers a complete solution for generating llama antibodies.
Overview
What are Single Domain Antibodies?
Single domain antibodies (sdAbs), also known as VHH antibodies, consist of a single binding domain from a heavy chain without associated light chain. This unique configuration in the world of antibodies confers several highly desirable properties. From a biotechnology perspective, sdAbs are easier to clone, engineer, incorporate or fuse with other antibody domains, for example, to create bispecific or trispecific antibodies. They are highly stable and can refold spontaneously following denaturation. Additionally, sdAbs express extremely well in various systems, including bacterial hosts and mammalian cells.
Their small size makes them attractive from a therapeutic standpoint. They penetrate tissues and cross natural barriers more easily than classical antibodies, and, thanks to their small size, can reach hidden epitopes located deeper within protein structures, such as deep grooves on virus surfaces or the catalytic sites of enzymes.
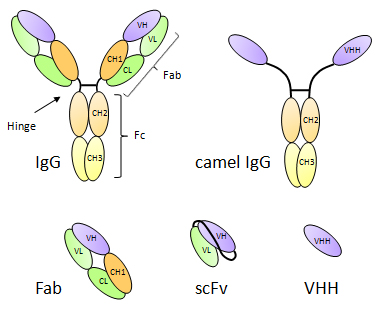
Heavy chain antibodies occur naturally in the blood serum of camelids (camels, dromedaries, llamas, alpacas, and vicuñas). Due to a genetic defect in isotype switching, VHH domains recombine directly with the CH2 domain of the IgG2 and IgG3 isotypes, bypassing the CH1 domain, which is lost in the process. This results in heavy chain antibodies that are 75 kDa homodimers, featuring two small VHH domains instead of two Fab fragments. It is often proposed that the compact size of VHH domain enhances viral immunity allowing them to bind crucial determinants located in deep crevices on viral surface.
Advantages of Single Domain Antibodies
- High affinity Small Size Antibody. Naturally occurring smallest known form of antibody, sdAb are only 13 kD and around 120 a.a. Their minimal size still allows for high specific with subnanomolar affinity.
- Good Physicochemical Properties. sdAb have a great stability with improved resistance to pH variations and heat. They can reversibly fold after heat-induced denaturation. sdAb are good candidates for intrabody development to target intracellular proteins and show great potential for oral availability.
- Downstream Engineering. Like antibodies, sdAb may require affinity maturation, humanization and proper developability. Their simple structure simplifies all these complex procedures, enabling fast track lead optimization. Since they are naturally single chain, they can be incorporated easily in higher structure (bi-specific, tri-specific antibodies). Naturally occurring sdAb are closely related to the human VH3 family, which is the most abundant VH family in humans, making humanization a relatively simple process.
- Small Drug Feature. With regard to targeting, development and administration. sdAb can target the active site of enzymes, receptor clefts and virus hidden epitopes; they can sustain harsher conditions (pH, heat) and be used in formats normally reserved for small drugs (e.g. oral administration).
- Enhanced Tissue Penetration. Better tissue penetration than IgG due to their small size; they can cross natural barriers and are actively under development for crossing the blood brain barrier and targeting the central nervous system. Inversely they also benefit from a better clearance, making them good candidates for tumor imaging.
- Similar to Human Antibodies. Camelid sdAbs are homologous to human VH3, which is the most prevalent VH family in humans.
- Easy Manufacturing. sdAb express very well on a variety of platforms (bacteria, yeast) and are cost-effective to manufacture on a large scale.
Generation of Single Domain Antibodies
S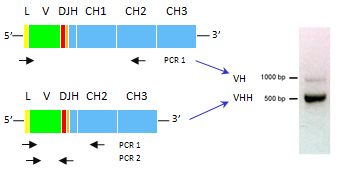 ingle-domain antibodies can be generated from llamas through a relatively simple and cost-effective process. Unlike traditional antibody generation, there’s no need to create combinatorial libraries of VH and VL domains. Instead, phage VHH libraries representing the immunized repertoire can be easily constructed from circulating B cells. VHH are rescued from the circulating PBMCs through a two-stage PCR where all the heavy chain cDNA are amplified up to the CH2 domain. After isolation of the smaller band containing the VHH, single domain antibodies are re-amplified and cloned in the phagemid vector.
ingle-domain antibodies can be generated from llamas through a relatively simple and cost-effective process. Unlike traditional antibody generation, there’s no need to create combinatorial libraries of VH and VL domains. Instead, phage VHH libraries representing the immunized repertoire can be easily constructed from circulating B cells. VHH are rescued from the circulating PBMCs through a two-stage PCR where all the heavy chain cDNA are amplified up to the CH2 domain. After isolation of the smaller band containing the VHH, single domain antibodies are re-amplified and cloned in the phagemid vector.
VHH Antibody Discovery
|
Procedure |
Description |
Timeline |
|---|---|---|
|
Immunization |
Standard immunization 12-weeks; short immunization 7-weeks procedure; custom immunization schedule, grouped immunization, dedicated animal available. Blood collection and PBMC preparation at bleed 2 and 3 (50 to 100 ml/bleed). Serum testing by ELISA. |
6-12 weeks |
|
cDNA Preparation |
Isolation of RNA & preparation of cDNA. |
1 week |
|
Library Construction |
Amplification of a large population of VHH. Cloning into any pADL™ phagemid, transformation, preparation of the phage library (>1E8 clones, >95% insert, >80% VHH). QC on 96 clones with full VHH sequence analysis, deep sequencing available. |
4 weeks |
|
Library Screening |
High avidity initial round, secondary selective rounds and competitive rounds using biotin/streptavidin and off-selection, custom selection available. Phage screen in 96-well format by ELISA, multiple ELISA conditions and competitive inhibition available. Phage validation by phage ELISA, DNA sequencing and direct binding assay using free VHH fragment. Deliverable free VHH fragment from best clones. |
4-6 weeks |
|
Production |
Cloning & large-scale expression of VHH. |
custom |
Immunization & Animal Handling
Our staff will assist you in determining the best immunization strategy compatible with your time frame. We offer different levels of work to our clients, including access to dedicated “naive” animals. PBMC purifications are processed immediately on site without delay after blood collection to preserve the quality of the mRNA and the high diversity of the library.
Construction of Immunized Single Domain Antibody Library
As one of the leaders in phage display technology, Antibody Design Labs offers all the tools necessary to build the best libraries. Our extended set of primers based on RACE sequencing covers many VHH genes, including sequences unreported in the IMGT database. Construction of libraries follows state-of-the-art procedure with a two-stage amplification of VHH fragments and cloning in any of the pADL™ phagemid vector series at a 1E9 library size.
Screening of Single Domain Antibody Library
T he high level of VHH expression usually prevents toxicity issues often seen with the display of Fab fragments and scFv. Good binders can be isolated within a limited number of rounds, although finding clones targeting the proper epitope can be challenging. We can accommodate custom screening tailored to your needs, from cell surface panning to competitive rounds.
he high level of VHH expression usually prevents toxicity issues often seen with the display of Fab fragments and scFv. Good binders can be isolated within a limited number of rounds, although finding clones targeting the proper epitope can be challenging. We can accommodate custom screening tailored to your needs, from cell surface panning to competitive rounds.
Additional Antibody Services
We offer multiple services to streamline the development of the best clones, from large-scale production to cloning and production into mammalian expression system of multi-speficic antibodies. Please, consult with our scientists to determine the best and lowest cost strategy.
Inquiry
Please, request a specific quote by completing the online quotation form accessible by pressing the following button; you will be contacted back by the next business day.
For all other inquiries, contact us by email at info@abdesignlabs.com or by phone at 1-877-223-3104 (PST). We guarantee competitive pricing. All our work is performed by highly experienced staff at our San Diego facility in California. All products and services are for research use only and are not intended for use in humans.

