ADL Phage Display Vector Series
Vectors for easy cloning, adaptable display and versatile applications.
Antibody Design Labs is offering a wide choice of phage & phagemid vectors to overcome the challenge of phage display. Selection of binders is a complex process depending on multiple variables and experimental conditions, making time and resources the main limiting factors for successful biopannings. We currently offer varied options for display on the N-terminal side of the full-length gene III protein with the PelB leader peptide either using a phagemid system or phage vectors. The phagemids are best suited for the display of antibodies such as scFv and Fab fragments but can also be used to display peptides at a low valency; phage vectors produce a multivalent display and are best suited for peptides and scFv. This short guide will help you with the choice of the vector that best suits your project.
-
Multiple tags and cloning sites.
-
Varied display strategies.
-
Alternative antibiotic resistance.
-
No background expression to maintain library integrity & stability.
-
Inducible expression & display.
-
“No MTA” attached.1
pADL™-10 – Low-Display Phagemid Vector Series
Phagemid pADL™-10b is our lead vector in this series. It uses a classical backbone with the high-copy number origin of replication pMB1, the f1 origin for packaging, a copy of LacI to insure proper repression of the lac promoter followed by a strong transcription terminator to prevent undesirable and toxic expression during cloning (Fig. 3). This configuration has been suggested to insure reliable cloning of antibody variable genes and improve library stability during screening (1). This vector is recommended for the display of scFv and is best suited when a step of recloning is anticipated for the expression of the insert in a soluble form.
The cloning site is a double BglI/SfiI sites which can accommodate very large libraries containing up to 10(9) to 10(10) transformants (Fig. 1). A 19 amino acid-long glycine-rich linker connects the cloning site to the first amino acid of the gene III protein. The absence of amber codon and the resistance to proteolysis of this linker result in robust display (Fig. 2). More on pADL-10b is available here.
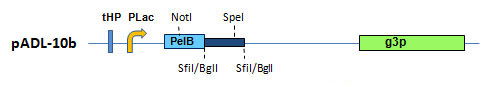
Figure 1. pADL-10 Phagemid Vector Series. Schematic representation of the cloning site.
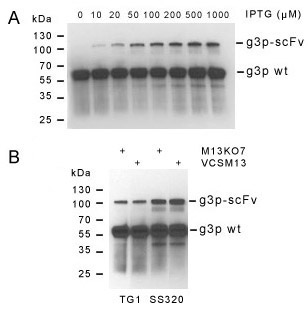
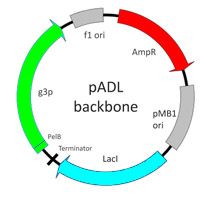
Figure 3. pADL-10 Backbone.
Figure 2. Control of Display. Western blot analysis of HyHEL-10 scFv displayed on gene III protein highlights some of the parameters controlling display with the pADL-10b vector. Induction of expression by IPTG strongly correlates with the intensity of scFv display (upper panel A). The amount of scFv on the phage shows little dependence on the helper phage, but is strongly influenced by the bacterial strain used to prepare the phage particles (lower panel B). Blots made with a gene III protein antibody.
pADL™-20 – High-Display Phagemid Vector Series
The phagemid pADL™-20 Vector Series uses an amber codon located before the start of the gene III protein to switch between display on the phage and expression of soluble antibodies (Fig. 4). All vectors possess a HIS tag for purification and varied tags for detection; direct secretion in the periplasm is obtained on non-amber suppressive bacterial strains and allows for direct binding analysis without a step of recloning. Different from pADL-10 vectors, phagemids in this series do not contain a copy of the lacI gene or the tHP terminator, but do have a stronger RBS to achieve higher levels of expression necessary for the display of both scFv & Fab fragments and a Lambda t1 terminator after the expression cassette to prevent excessive expression of the beta-lactamase and rapid consumption of ampicillin when maximum induction for periplasmic expression is reached.
The level of expression of the newer c series phagemids is equivalent to the level of expression of pCOMB3 phagemid and is significantly lower than pHEN1 or pHEN2 phagemids (Fig. 5). Excessive expression results in toxicity (Fig. 5, Lane 6), making antibody libraries genetically unstable and favoring strong bias toward loss of diversity. In this regards, pADL-20 series c phagemids provides a well-balanced expression between toxicity & display and strong expression of free antibody in the periplasmic space on non-amber suppressive bacterial strains, as pCOMB3 phagemid has proven to do.
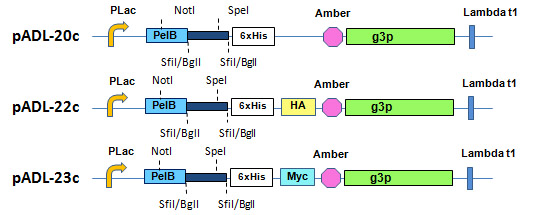
Figure 4. pADL™-20 Phagemid Vector Series. Schematic representation of the cloning site.
More details on the cloning site can be found here for pADL-20c, pADL-22c, and pADL-23c, respectively. Preparation of virions will also require a helper phage; we recommend CM13, which can be found here.
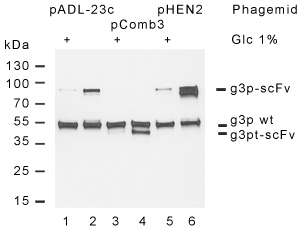
Figure 5. Comparative analysis of pADL-23c display. Display of the anti-lysozyme HyHEL-10 scFv was analyzed by Western blot with a g3p antibody. Phagemids pADL-23c (lane 1 & 2) and pCOMB3 (labe 3 & 4) give a similar level of display, around 25% measured as the percentage of fusion to the total amount of g3p protein, while pHEN2 (lane 5 & 6) exhibits a much higher level, close to 75%. A strong band under the expected size indicates that a majority of scFv displayed by pHEN2 is proteolyzed while pADL-23c and pCOMB3 show little proteolysis. Briefly, HyHEL-10 scFv was cloned between the PelB leader sequence and the full-length g3p in both pADL-23c & pHEN2 and between the OmpA leader sequence and the truncated g3p in pCOMB3; virions were produced using TG1 cells and CM13 helper phage after an 8 h incubation at 30°C in absence of glucose and purified by PEG precipitation. Bacterial growth was robust for pADL-23c & pCOMB3 and very slow for pHEN2. Virion production was higher for both pADL-23c & pCOMB3 as well. Similar results were obtained through multiple experiments. Abbreviations g3pwt: wild-type g3p; g3p-scFv: fusion between HyHEL-10 scFv and g3p; g3pt-scFv: fusion between HyHEL-10 scFv and truncated g3p.
pADL™-23Chlo – Alternative Antibiotic Resistance
The phagemid pADL™-23Chlo is a variant of the successful pADL™-23c phagemid with a chloramphenicol-resistance marker. Having access to an alternative antibiotic resistance has multiple advantages. Contaminations by positive control phage is a difficult challenge. The screening of contaminated libraries often result in the isolation of the control rather than new binders. The use of an alternative selection marker allows to clone and study the display of positive controls without fears of a positive contamination. Other advantages include the use of a better selection marker to improved stability of complex libraries or the ability to build intermediate libraries with limited cross-contamination from the parent library.
More details can be found here for pADL-23Chlo.
pADL™-43 – Display on Truncated pIII
The phagemid pADL™-43 is a variant of pADL™-23c phagemid with a truncated pIII protein. The displayed moieties are fused to the C-terminal domain of pIII, resulting in smaller fusion proteins more amenable to the display of large proteins. pADL-43 is similar to pComb3, which is know to favor the display of Fab fragments. In our hands, pADL-43 displays Fab fragments on the same level as pADL-23c, but gives a better binding to low affinity binders, likely as a result of higher local density of the fusion proteins.
More details can be found here for pADL-43.
pADL™-100 – Phagemid Vector Series without Amber Codon
The phagemid pADL™-100 Vector Series combines features known to maximize phage display. These include a strong RBS, a proteolysis-resistant linker and no amber codon. Amber codons are only partially suppressed in vivo, resulting in limited synthesis of full-length pIII fusions required for incorporation in the phage structure and display. The pADL-100 phagemid vector was designed for applications requiring the highest levels of display such as peptide phage libraries. Display can be further enhanced by the use of the pIII-defective helper phage M13KO7d3; more on this helper can be found here.
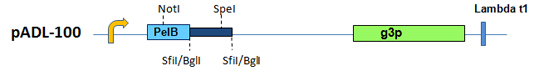
Figure 6. pADL™-100 Phagemid Vector Series. Schematic representation of the cloning site.
More details on the cloning site can be found here for pADL-100.
fADL™ – Multivalent Phage Vector Series
Phage vectors do not require the use of helper phage and can be propagated in F- strains, making them interesting alternatives to phagemids for building phage libraries. Phage vectors are best suited to display peptides thanks to their inherent multivalency but are also very effective in displaying antibodies (7).
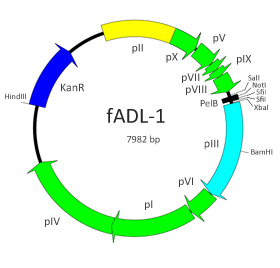
Figure 7. fADL-1 Backbone.
We have engineered the phage vector fUSE5 (4) by exchanging the tetracycline cassette with a kanamycin resistance gene and replaced the natural gene III leader peptide with the PelB leader (Fig. 7 & 8); the result is the phage vector fADL™-1 that is smaller in size and compatible with most phagemid vectors for cloning. The display of scFvs by fADL-1 is intense and multivalent (see here).
fADL™-1. First vector of the series, now discontinued.
fADL™-1e. A variant of fADL-1 with an improved cloning site and a mutation in pVIII that is found in M13.
fADL™-2blue. fADL-2blue is the most recent vector of the series and has several improvements over the original fADL-1. Elution can be done by trypsinization, a technique that improves recovery (8). The selection is done by ampicillin, which prevents the co-selection of kanamycin-resistant helper phage such as M13KO7 and derivatives. A blue color in the presence of X-gal and IPTG by α-complementation of the β-galactosidase further differentiates potential contaminations. Finally, the minus origin of replication has been further deleted on the 3′ side, preventing reversion to wild type phage in absence of selection, making the vector overall more stable.
Historically, the vector fUSE5 was used to build the first peptide library (4). fUSE5 is a derivative of the fd-tet vector created by George Smith (2). The fd-tet vector contains a tetracycline cassette near the minus-strand origin that imposes a rate-limiting growth on all clones with the benefit of more stable libraries (3). Since then, many libraries either peptides or antibodies (5) have been build with phage vectors derived from fd-tet. We found that not only scFv are very-well displayed by fd-tet derivatives but with very limited polyphage formation to the contrary of virions produced with helper phage defective in gene III protein, a classical means to improve antibody display (6).

Figure 8. Sequence of fADL-1 cloning site.
Notes
1 Antibody Design Labs grants to the buyer with the sale of its phage and/or phagemid vectors (the “Product”) a non-exclusive, non-transferable, royalty-free, commercial license to use Product in research conducted by the buyer (whether the buyer is an academic or a for-profit entity). The buyer is NOT granted a license to (a) use Product for human or animal therapeutic, diagnostic, or prophylactic purposes, (b) act as reseller or distributor of Product, or (c) resell, distribute, or transfer Product without modification under any name. Antibody Design Labs does not warrant that the use or sale of Product, the use thereof in combination with other products, or the use of Product in the operation of any process will not infringe the claims of any United States or other patent(s). If the buyer is not willing to accept the limitations of this license, without modification, buyer may refuse this license by returning Product unopened and unused. By keeping or using Product, buyer agrees to be bound by the terms of this license.
References
-
Krebber, A., Bornhauser, S., Burmester, J., Honegger, A., Willuda, J., Bosshard, H. R., & Pluckthun, A. (1997). Reliable cloning of functional antibody variable domains from hybridomas and spleen cell repertoires employing a reengineered phage display system. J Immunol Methods, 201(1), 35–55.
-
Zacher 3rd, A. N., Stock, C. A., Golden 2nd, J. W., & Smith, G. P. (1980). A new filamentous phage cloning vector: fd-tet. Gene, 9(1-2), 127–140.
-
Thomas W.D., Golomb M., Smith G.P. (2010). Corruption of phage display libraries by target-unrelated clones: diagnosis and countermeasures. Anal. Biochem., 407(2):237-40.
-
Scott J. K. & Smith G. P. (1990). Searching for peptide ligands with an epitope library. Science, 249(4967):386-90.
-
Cai X. & Garen A. (1997). Comparison of fusion phage libraries displaying VH or single-chain Fv antibody fragments derived from the antibody repertoire of a vaccinated melanoma patient as a source of melanoma-specific targeting molecules. Proc. Natl. Acad. Sci. USA, 94:9261–9266.
-
Rondot S., Koch J., Breitling F., Dübel S. (2001). A helper phage to improve single-chain antibody presentation in phage display. Nat. Biotechnol., 19(1):75-8.
-
O’Connell, D., Becerril, B., Roy-Burman, A., Daws, M., & Marks, J. D. (2002). Phage versus phagemid libraries for generation of human monoclonal antibodies. J. Mol. Biol., 321(1), 49–56.
-
Thomas, W.D., Smith, G.P. (2010). The case for trypsin release of affinity-selected phages. Biotechniques, 49(3):651-4.
PUB004 – Rev230812
Please send comments to info@abdesignlabs.com.
Copyright © 2023 Antibody Design Labs. All rights reserved. The reuse or reproduction of any of the information, design or layout contained in this web site without the permission of Antibody Design Labs is prohibited.
