Catalog
Recently Viewed
pADL-20c
Technical Description
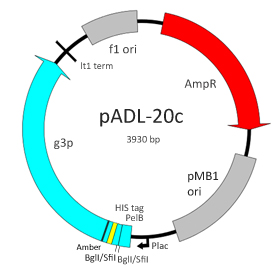
pADL™-20c is a phagemid vector designed for phage display on the N-terminal side of the protein III of the filamentous bacteriophage M13 or equivalent. This vector contains a PelB leader sequence for expression in the periplasm, a double-SfiI/BglI cloning site to introduce scFvs or Fab fragments, a HIS tag for purification and an amber codon located before the full-length copy of the gene III sequence. On non-suppressive bacterial strains, free scFvs or Fab fragments are produced in the periplasm where they can be assayed for binding or purified for further testing. Expression of the fusion is under the control of a lac promoter that has been adjusted at a level comparable to the widely used phagemid pCOMB3.
Applications
-
Phage display at the N-terminal side of gene III protein of the filamentous bacteriophage.
-
scFv and Fab display.
-
Free scFv or Fab expression.
For research use only; not intended for any animal or human therapeutic or diagnostic use.
Specifications
General Characteristics:
Plasmid Size: 3930
Promoter: lac promoter
Leader Peptide: PelB
Cloning Site: double-BglI/SfiI, NotI-SpeI
Purification: HIS tag
Fusion Protein: full length gene III protein and conditional amber stop codon
Selection: ampicillin
Replication: oriF1, pMB1
Physical Characteristics:
Concentration: 0.5 µg/µl.
Product Size: 10 µg.
Buffer: DNA Conservation Buffer (Tris/HCL 5 mM, EDTA 0.1 mM, pH 8.5, sterile).
Storage Temperature: -20°C.
Quality Control & Certification of Analysis
Product Size:
Digestion by EcoRI and NheI generates 2 fragments of 2.6 kb and 2.0 kb.
Product Sequence:
Complete vector sequence is analyzed.
Certification:
Product meets all specifications.

Citations
-
Jeong SL, Zhang H, Yamaki S et al. (2022). Nucleic Acids Res.,2:gkac995.
-
Stefan M.A., Light Y.K., Schwedler J.L., McIlroy P.R., Courtney C.M., Saada E.A., Thatcher C.E., Phillips A.M., Bourguet F.A., Mageeney C.M., McCloy S.A., Collette N.M., Negrete O.A., Schoeniger J.S., Weilhammer D.R., Harmon B. (2021). Development of potent and effective synthetic SARS-CoV-2 neutralizing nanobodies. MAbs. 13(1):1958663.
-
Huo J., Le Bas A., Ruza RR. et al. (2020). Neutralizing nanobodies bind SARS-CoV-2 spike RBD and block interaction with ACE2. Nat Struct Mol Biol., 27(9):846-854.
-
He L., Lin X., de Val N., Saye-Francisco K.L., Mann C.J., Augst R., Morris C.D., Azadnia P., Zhou B., Sok D., Ozorowski G., Ward A.B., Burton D.R., Zhu J. (2017). Hidden Lineage Complexity of Glycan-Dependent HIV-1 Broadly Neutralizing Antibodies Uncovered by Digital Panning and Native-Like gp140 Trimer. Front Immunol., 8:1025.
-
WIPO Patent Application (2016). Method For The Treatment Of Malignancies. WO 2016/154473 A1.
-
WIPO Patent Application (2016). Camelid Single-domain Hcv Antibodies And Methods Of Use. WO 2016/172121 A1.



